Source: administrator
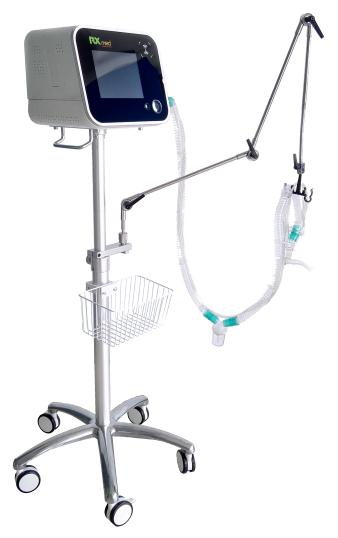
RUXIN(BEIJING)MEDICAL SYSTEMS CO., LTD.(Runxin Technology), after unremitting efforts with the support and the help of many parties, research & development-produced the CoughSync100 synchronous expectoration machine, On July 1, 2020, Runxin obtained “People’s Republic of China Class III Registration Certificate for Medical Device” issued by National Medical Products Administration (National Machinery registration 20203080594).
The acquisition of the certificate means that the CoughSync100 synchronous expectoration machine has officially launched the promotion and application in the domestic market, which also is seeing as a milestone for the improvement of the domestic ICU airway management technology level.
On July 1, 2013, Runxin Technology introduced the proprietary technology of the CoughSync100 synchronous expectoration machine from Innovent Israel and signed a global exclusive cooperation agreement with Innovent, which Started the technology commercialization operation with the new technology as Runxin’s main body.
The CoughSync100 synchronous expectoration machine is the world's first device to help patients with ICU invasive mechanical ventilation that perform automatic, non-invasive sputum drainage device. The device works in parallel and synchronously with the ventilator. It does not affect the normal use of the ventilator and can replace most of the invasive manual sputum suction operation commonly used in the current ICU clinical airway cleaning.
CoughSync100 not only provides a safer and more efficient airway clearance technique for ICU invasive mechanical ventilation patients and clinical medical staff, but also heralds the emergence of a new segment of the ICU respiratory management market. At the same time, it also provides clinical experts with a more valuable physical method option to solve the problem of drainage of sputum and sputum plugs in the small airways in the lungs with no effective means.